There are a few key points that should be considered with the Mass Spectrometers and how they are able to detect the molecules of interest.
- The detection mode
Molecules are ionized by the mass spec, in other words, given a charge based on the chemical structure of the molecule. Positive mode means that the molecules are typically gaining an additional proton while negative mode results in the loss of a proton. We use liquid nitrogen to feed the molecules into the machine.
The mass specs in the Facchini lab are all set to positive mode as that is optimum for the poppy BIAs that the lab has previously processed and alkaloids in general with the nitrogen protonation. In contrast, we lose some fundamental molecules without further processing. Alcohol groups (OH) are better detectable in negative mode. However, there are numerous molecules that end up being zwitterions and will not deal well in either mode.
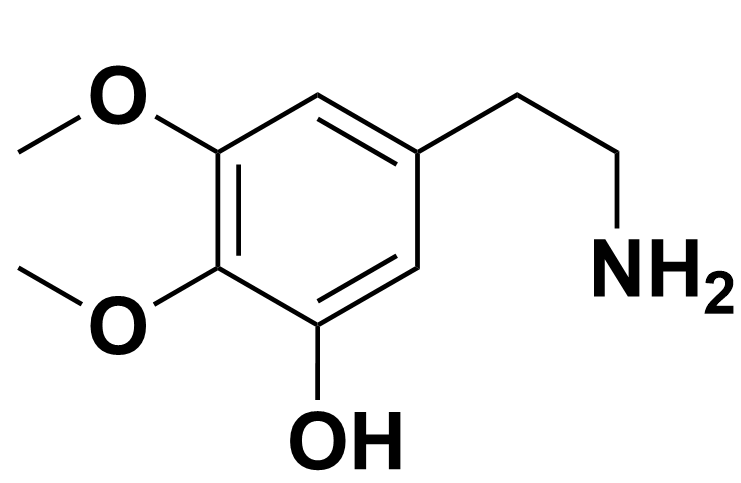
For example, 3,4-dimethoxyl-5-hydroxyphenethylamine is an intermediate step to form the cacti psychedelic, mescaline. This will result in a zwitterion when the OH group is deprotonated to form O- and the N is protonated into NH3+.
In these cases with untargeted metabolites, it will really need to be optimization of the most important set of molecules and compromise on the rest.
- Solvent Systems
The solvents used in the mass spectrometer can partially help maintain the charge. There are two solvents used to establish the gradient. Typically, for the poppy alkaloids, solvent A is water with 0.1% formic acid, while solvent B is acetonitrile with 0.1% formic acid. Both solvents are polar in different "strengths" to create a difference in gradient to help with elution. The formic acid in both solvents will drop the pH to 2-3 so protonation is encouraged.
Solvent system gradients can also be adjusted for. Shallower gradients where solvent A is ran through the machine at a more gradient slope can help separate peaks that are clumped together to resolve them better. In a 12 minute run, this can look like 0-60% solvent A from 0-9 minutes, then 60-100% from 10-12 minutes. A shaper gradient could be zero to 100% solvent A in a 3 minute duration.
- Columns
Columns are filled with material to help the separation of molecules. The Facchini lab uses a C18 column which are a collection of 18 carbon-carbon chains.
In combination with our polar solvent system and non-polar C18 column (stationary), this is a reverse phase system. More conventional chromatography uses a non-polar solvent system and a polar stationary phase, as is the case with hexane solvents and silica Thin Layer Chromatography plates.
Reverse phased systems mean that the polar/positive ions in the plant samples are eluted further than the non-polar samples.
We typically use complex plant samples without further refinement. This may lead to complications called matrix effects and ion suppression. Ideally, one molecule passes at a time into a column to have clear separation. In a complex sample, that is difficult where multiple metabolites can pass through the column at the same time. This interaction is a matrix effect where molecule A can influence molecule B as it passes through the column. This can change the retention time and interaction with the C18 column.
Ion suppression is a similar situation where one molecule can influence the signal of another to decrease the amount detected by the machine.
Here is an example chromatogram, particularly a EIC or exact ion count. It has isolated the particular mass that I specified it to be.
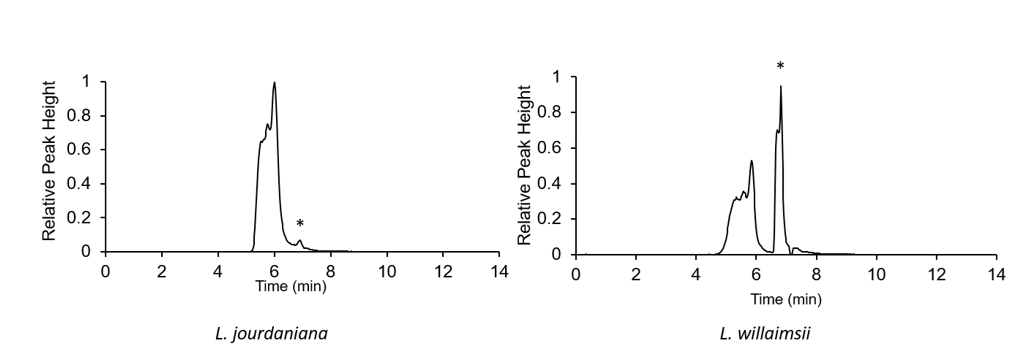
It is not possible to make a qualitative conclusion based on the integrated peak areas from the mass spec without a standard curve to compare it to.